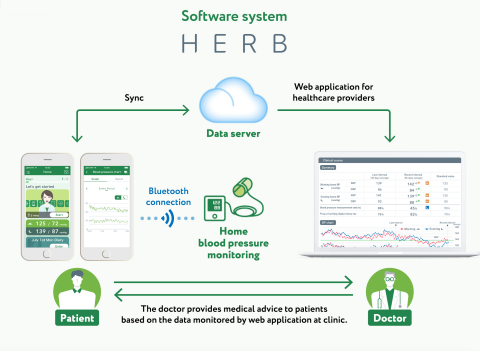
KUALA LUMPUR, Sept 13 (Bernama) — The results of a Japanese Phase III domestic multicenter randomised controlled trial of a digital therapeutic app for hypertension (DTx) jointly developed by CureApp Inc (the Company) and Jichi Medical University were presented recently.
It was presented by Professor Kazuomi Kario of Jichi University’s Department of Cardiovascular Medicine at the European Society of Cardiology Congress held at the end of August 2021 (ESC Congress 2021 – The Digital Experience: Late Breaking Trials in Hypertension).
The results of this trial were also published in the European Heart Journal, one of the world’s leading journals on cardiovascular disease, according to a statement.
This clinical trial ran from January 2020 to December evaluating the efficacy and safety of the therapeutic app for patients with essential hypertension.
This trial was performed as a comparative study of two groups, a control group which received guidance on lifestyle changes based on the Guidelines for the Management of Hypertension 2019 (the Guidelines), and an intervention group which used this therapeutic app in addition to applying lifestyle changes in accordance with said Guidelines.
This represented the world’s first trial of a therapeutic app for the purpose of regulatory approval in the field of hypertension.
The difference in the 24-hour systolic blood pressure between the groups (adjusted mean value) based on ABPM at the study entry point of 12 weeks – the primary evaluation item – was -2.4 mmHg, indicating a significant hypotensive effect in the intervention group using the therapeutic app compared to the control group.
The control group was provided guidance on lifestyle changes, and had their blood pressure monitored every day.
A difference in the 24-hour systolic blood pressure between the groups based on ABPM of -2.4 mmHg represents a clinically significant change, reducing the risk of developing cardiovascular diseases and cerebrovascular diseases by 10.7 per cent.
The 10 mmHg reduction of morning home SBP provided when using this therapeutic app demonstrates a clinically significant outcome in reducing cardiovascular diseases, while also demonstrating the effectiveness of the therapeutic app as an option for treating hypertension in the future.
CureApp Inc is a medtech startup that conducts research and development and manufactures and distributes software medical devices. More details at http://cureapp.co.jp/
— BERNAMA
